Randomized Clinical Trials: Design, Practice and Reporting
Free download. Book file PDF easily for everyone and every device. You can download and read online Randomized Clinical Trials: Design, Practice and Reporting file PDF Book only if you are registered here. And also you can download or read online all Book PDF file that related with Randomized Clinical Trials: Design, Practice and Reporting book. Happy reading Randomized Clinical Trials: Design, Practice and Reporting Bookeveryone. Download file Free Book PDF Randomized Clinical Trials: Design, Practice and Reporting at Complete PDF Library. This Book have some digital formats such us :paperbook, ebook, kindle, epub, fb2 and another formats. Here is The CompletePDF Book Library. It's free to register here to get Book file PDF Randomized Clinical Trials: Design, Practice and Reporting Pocket Guide.
Contents:
Types of Trial Designs
Our courses. HOME Study recruits first patients! Would you like to join our team? Largest-ever clinical trial in hip fracture fixation reaches key milestone Dietary fibre metabolite helps immune system fight invasive bacteria New Cochrane review on exercise for prevention of falls in older people Arterial Revascularisation Trial published Pills as good as injections to treat bone and joint infections, paper finds OVIVA and FAIT Trials Published Study suggests immune system plays key role in survival after pancreatic cancer surgery New drug class could offer a targeted safer treatment alternative for patients with rheumatoid arthritis Kennedy Institute launches Oxford Centre for Microbiome Studies Risks of shoulder replacement surgery higher than previously thought Genes and height matter for carpal tunnel syndrome First patient recruited to TWO Study!
ORiF opens for recruitment!
GRASP trial completes recruitment! Study reveals new drug target for rheumatoid arthritis Key link discovered between tissue cell type and different forms of arthritis HOPE-e opens for recruitment! Mounting evidence that BMI and smoking should not be used for rationing knee and hip replacement How do you explain your research to the public? NINJA trial completes recruitment!
For clinical trials, reporting guidelines, such as the Consolidated Standards of Reporting Trials CONSORT , 1 and CONSORT extensions, such as for reporting noninferiority and equivalence trials 2 or multiple-group trials, 3 provide a useful framework for presentation of the main elements of trial methodology and results. A key principle is that the published article is intended to represent a faithful scientific record of the clinical trial. The report needs to follow the study protocol and statistical plan as prespecified or formally amended, with strong justification for deviations.
The report does not represent the study the editors or external peer reviews would have preferred the investigators had designed. Equally important is that the report of the trial does not represent the study other methodologists and statisticians would have designed given their preference, even if their community may be moving in directions different from when the trial was formalized. The obligation of the journal is to report, not to modify, the trial, although in some cases, it may be reasonable for reviewers and editors to request additional analyses to supplement and clarify the prespecified analytic approach.
It is also important for researchers and readers to recognize that the article is not the study.
Randomized Clinical Trials: Design, Practice and Reporting - Semantic Scholar
The report of the trial can only be a limited representation of the study in the same way that a photograph of a body is not the body, and even a body is only a representation of a person. While appended protocols and statistical analysis plans can provide details about the study design, it is impossible for the article to fully convey what occurred during the execution of the trial, and impractical for the article to present all the raw data collected or even, usually, all the analyzed data. The published article represents a formalized record of the study, with limitations in what can be communicated even if article length were not a barrier.
Moreover, if the article is only a summary of the study, the Abstract is but a skeleton of the article. The Abstract cannot provide sufficient detail to critically appraise the study for validity, and for studies that have any complexity the Abstract should not be used as the basis for nuanced interpretation, even if caveats are included. Some trials have straightforward results, such as those that have very little dropout of enrolled participants, no early termination, clinically meaningful end points, unambiguous primary results, and reasonably consistent secondary end points.
When this is not the case, it is responsibility of the journal editors to make sure that the challenging issues are clearly conveyed and that the nuances are objectively addressed in the Discussion section of the article. As with all fields of science, the sciences of study design and statistics are in constant evolution.
In many cases such trials provide the key evidence necessary for the regulatory approval of a new product for future patient use. Randomized Clinical Trials. Using examples and case studies from industry, academia and research literature, Randomized Clinical Trials provides a detailed overview of the key issues.
Methods that were considered standard and important at the time a trial was designed may no longer be favored by the time the trial is finished, particularly for trials that take many years to complete. As one example, for an older trial last observation carried forward may have been the acceptable and prespecified approach to handling missing data at the study inception, but this approach has generally been superseded by other techniques such as multiple imputation, when appropriate.
Why clinical trial outcomes fail to translate into benefits for patients
Other methods such as Bayesian statistics have gained traction but have not become widely embraced as a new standard. These arguments acknowledge that the most commonly used significance threshold of. Others have continued to advocate for describing results of clinical trials in terms of statistical significance, in part because of the need for a starting point in discussion; decisions that are made by regulatory bodies, such as the US Food and Drug Administration, which are generally dichotomous; and the need to assist clinicians and patients in their interpretation and operationalization of clinical trial results.
Over time, the research community comes to a consensus about preferred methods—a consensus that may last only until the next set of methods are developed. As the community evolves, the newer methods become integrated into study design and then appear with increasing frequency in the studies submitted to the journals. For example, when alternatives to hypothesis testing using significance thresholds become established, they will be incorporated into the study from inception.
When this process occurs, those methods become part of the scientific report. However, at times, journals may publish post hoc analyses that use other or newer methods that may be as and in some cases, perhaps more informative than the preplanned design, although these reports should include proper context for the new analyses and appropriate caveats.
For example, a post hoc Bayesian reanalysis of a previously published RCT that compared the effects of venovenous extracorporeal membrane oxygenation ECMO vs conventional mechanical ventilation on mortality among patients with severe acute respiratory distress syndrome was performed because of divergence between the clinical vs statistical significance of the trial findings and continued controversy over the benefit of ECMO.
Clinicians may have the most challenging role in understanding the results of RCTs because they have to take the necessarily incomplete information provided in the scientific report, decide whether it is potentially actionable, and, if so, decide whether and how the findings can be applied to individual patients. An Editorial that puts the study in context of other research, delves into the clinical implications, and highlights the limitations in study interpretation can be helpful for readers in interpreting the study.
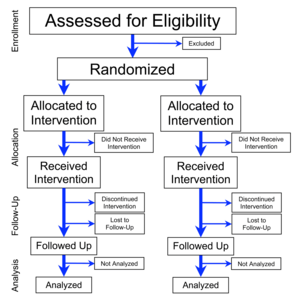
For clinical trials submitted to JAMA , authors are required to ensure that the study design and results are reported with fidelity to and consistency with the a priori decisions that investigators made in designing their trials, as prespecified and documented in the trial registration, study protocol, and statistical analytic plan.
The editors review these documents carefully for consistency and to ensure that authors provide explanations and justification for any differences among these documents.
- Pop Goes the Weasel (Alex Cross Novels).
- Trigonometry.
- Best Practice in Design, Analysis and Reporting of Trials.
- HI FRIEND!.
- ADVERTISEMENT!
- References!
Investigators declare in those documents the hypotheses being tested in the trial, the primary outcome the study is designed to examine, and the statistical criteria that allow investigators to evaluate the probability or implausibility of observing the results obtained. For trials in which investigators use a frequentist approach the most common approach to statistical inference in the current clinical literature in the design and analysis, the reporting of results should reflect that approach, including interpretation based on the prespecified effect size estimate, the sample size anticipated vs achieved , and the criteria and threshold for declaring statistical significance.
For studies in which hypothesis testing involves prespecified use of P values, those values should be reported with the study results, along with confidence intervals for estimates of effect size. For trials in which investigators designed the study using a Bayesian approach or other evolving designs rather than a frequentist approach to analysis, the reporting of the results should be consistent with the prespecified analytic plan and follow accepted norms for that analytic approach.
The presentation and interpretation of the results from most clinical trials are usually straightforward. The results for the prespecified primary outcome take priority and precedence over all other outcomes, should be the focus of the submitted manuscript, and should be reported in detail. For instance, in a parallel-group trial, results usually include reporting of absolute event rates for the primary outcomes for the study groups, with between-group differences and confidence intervals reflecting the estimated uncertainty around the effect size, and risk ratios, as appropriate.
The primary outcome forms the basis for the Discussion section and for the Conclusion of the article. Clinical trials also include prespecified secondary analyses and outcomes, subgroup analyses, exploratory outcomes, and post hoc analyses. These findings are commonly included in reports of clinical trials but must be presented appropriately, objectively, and in context.
The art of quality assessment of RCTs included in systematic reviews. Randomized Clinical Trials David Machin. Sign up to take part. The effect of hydro alcoholic nettle Urtica dioica extracts on insulin sensitivity and some inflammatory indicators in patients with type 2 diabetes: A randomized double-blind control trial. Sexual Med. With key features such as key terms, people and places, Facts gives you all the information you need to prepare for your next exam. If you only want to read and view the course content, you can audit the course for free.
Authors should indicate the number of secondary outcomes and how many are being reported in the manuscript. The methods and analyses on which these findings are based should be detailed in the Methods section of the manuscript, and the findings should be reported in the Results section and should be clearly designated according to prespecified definitions and analytic plans; they should be presented in an organized, logical, and consistent fashion. With analyses of outcomes beyond the primary outcome, it is essential that appropriate prespecified analytic strategies are in place to account for type 1 error due to multiple comparisons; interpretation of these outcomes must address the possibility of false discovery and erroneous inferences, and acknowledge that these observations may need to be considered exploratory.
Despite the prominent position of the Abstract in a research article, the inherent space limitations necessitate that the Abstract of the report of an RCT be considered only a presentation of the key points in study design and the key study results. Clinical Rehabilitation, 29 1 , When the groups that have been randomly selected from a population do not know whether they are in the control group or the experimental group.
Being able to show that an independent variable directly causes the dependent variable. This is generally very difficult to demonstrate in most study designs. Confounding Variables. These variables render it difficult or impossible to distinguish the relationship between the variable and outcome being studied. A relationship between two variables, but not necessarily a causation relationship.
Welcome to the CONSORT Website
When the researchers conducting a blinded study do not know which participants are in the control group of the experimental group. That the relationship between the independent and dependent variables the researchers believe they will prove through conducting a study does not exist. To "reject the null hypothesis" is to say that there is a relationship between the variables. A group that shares the same characteristics among its members population. A sample may be skewed by those who are selected or self-selected into a study.
- Ruby Slippers, Golden Tears (Fairy Tale Anthology, Book 3)
- Estrogen — Mystery Drug for the Brain?: The Neuroprotective Activities of the Female Sex Hormone
- Home Security Projects for Arduino
- Pragmatics and Law: Philosophical Perspectives
- The Lost Art of Being Happy: Spirituality for Sceptics
- The Bioarchaeology of Individuals
- Trigonometry