Embryonic Stem Cells (Human Cell Culture)
Free download. Book file PDF easily for everyone and every device. You can download and read online Embryonic Stem Cells (Human Cell Culture) file PDF Book only if you are registered here. And also you can download or read online all Book PDF file that related with Embryonic Stem Cells (Human Cell Culture) book. Happy reading Embryonic Stem Cells (Human Cell Culture) Bookeveryone. Download file Free Book PDF Embryonic Stem Cells (Human Cell Culture) at Complete PDF Library. This Book have some digital formats such us :paperbook, ebook, kindle, epub, fb2 and another formats. Here is The CompletePDF Book Library. It's free to register here to get Book file PDF Embryonic Stem Cells (Human Cell Culture) Pocket Guide.
Contents:
Though it makes culture maintenance difficult, ESC differentiation is a good thing, because it indicates a healthy culture. A trouble-free culture should raise flags. If you're picking-to-keep often, says Manning, you're probably doing something wrong. So therefore, you should never hit over half being differentiated. If you find yourself picking to keep, say more than once every other month, maybe once every three months You should be doing pick-to-remove, not pick-to-keep. Picking too frequently can cause abnormal cell outgrowth, so it's important not to be a perfectionist, says Manning. Besides, they could adversely affect your culture.
Those antibiotics could select for abnormal cells for all you know. Though WiCell's protocols pertain directly only to its lines, chances are good they pertain to you, too. Of the 22 federally approved hESC lines, five come from the Wisconsin Alumni Research Foundation, which established WiCell in to, among other things, distribute those lines to researchers around the world. WiCell distributes cells between passage 20 and 25 that have been karyotyped to ensure they are genetically normal.
- Cost Management: Accounting and Control, 6th Edition?
- Word-Formation in English (Cambridge Textbooks in Linguistics)?
- Legal Principles in WTO Disputes?
- Memory Detection: Theory and Application of the Concealed Information Test?
- References.
But you should have them karyotyped, too, says Manning. Then, when you start getting really great data, it's good to check before you finish, and again before you publish. We also recommend you karyotype every 10 passages after passage Build up an emergency supply of frozen cells as soon as possible after thawing. Says Manning, "once you're at the point of expanding, start freezing, a few vials at a time. You want to start freezing as soon as possible, because you want the lowest passage cells. Don't wait until you have lots of cells to start freezing.
That's when the cells are growing most rapidly and will be thaw most robustly. Join senior editor Jeffrey Perkel as he learns how to culture stem cells. Manning stresses that hESC culture cannot realistically be a side project.
Tricks For Human Embryonic Stem Cells
Between culturing MEFs and splitting and maintaining the hESCs, the culture work consumes more than 20 hours per week. As a result, it's critical that you train another person to cover your culture needs in the event you are sick or just need a vacation. Using a sterile glass pipet, add the appropriate amount of warmed hES cell culture medium to each well. For cultures in a 6-well plate, add 2. Transform 9 inch glass Pasteur pipettes into picking tools by molding the tips over the controlled flame of an alcohol burner. Be sure that the tip of the pipette is sealed to prevent contamination and rounded to avoid scratching the plastic of the culture dish.
Sterilize the picking tools before use using the UV light source in the biological safety cabinet or picking enclosure. Remove the differentiated areas. Differentiation often occurs in only a portion of an hES cell colony, usually along the edges or as isolated spots in the center. If only a section of a colony is differentiating, only the differentiated area needs to be removed. Differentiation can often lift off the plate in a "sticky" sheet, and it is helpful to first separate the differentiated cells from the rest of the colony by drawing a line through the colony with the end of the picking tool.
Once the pieces of the colony are separated, gently glide the picking tool along the plate to detach the unwanted cells.
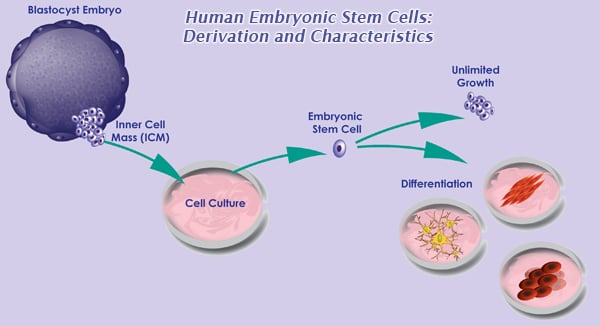
Culture and Maintenance of Human Embryonic Stem Cells
Take care not to scrape away too much of the MEF feeder layer between the colonies in this process. When all of the differentiated cells are removed from the plate and floating in the medium , return the culture to the biological safety cabinet. Aspirate the medium containing the differentiated cells and replace with fresh hES cell culture medium. In a sterile biological safety cabinet, remove the lid of the 6-well culture dish and aspirate the spent medium from the wells to be passaged.
Using a sterile glass pipet, add 1 mL of warmed collagenase IV to each well to be passaged. After incubating the cells with collagenase, observe the colonies under the microscope. The enzyme should cause a subtle but observable change in the colony edges. In the biological safety cabinet, gently aspirate the enzyme from each well and replace with 2.
Tip the culture plate slightly toward you and take up the 2. Holding the pipet perpendicular to the bottom of the plate, gently glide the pipet tip across the well while slowly releasing the medium. Repeat the scraping and pipetting motion times until all of the colonies have been removed from the well. Pipet gently to avoid breaking up the colonies into too small of pieces. When the cells have been removed from the first well, leave the contents in the well and begin scraping the cells off of the next well.
When all of the wells to be passaged have been scraped, pool the medium containing the colony pieces from each well in a sterile 15 mL conical tube. Rinse the scraped wells by adding 1 mL of hES cell culture medium to each well. Collect this rinse and transfer to the conical tube.
- Popular Products.
- Embryonic stem cell!
- Tricks For Human Embryonic Stem Cells | The Scientist Magazine®.
Pipet the cells gently in the tube to break up the colony pieces up to the desired size. Label the plate with the appropriate hES cell information: cell line, new passage number, and passage date. Aspirate the MEF medium from the wells and add 1. Gently swirl the buffer around the wells and aspirate the PBS wash. Add 1. When the hES cells are finished centrifuging, bring the tube back to the biological safety cabinet. Aspirate the supernatant, being careful not to disturb the loosely-packed cell pellet. Resuspend the cells in the pellet with enough medium for 1 mL hES cell culture medium per well to be plated.
When splitting into 6 new wells, resuspend the pellet in 6 mL medium.
Gently and evenly add 1 mL of the cell suspension to each prepared well of the MEF feeder plate. Undifferentiated hES cells are small, tightly-packed, and usually consist of larger, more spread out cells.
Services on Demand
Differentiation can occur within a colony Figure 1A or between colonies Figure 3B. Different morhopologies can be seen under low magnification under the microscope. A good cell morphology, as seen in Figure 2A, contains small, tightly packed cells that grow in a monolayer.
The greatest therapeutic promise of human embryonic stem cells (hESC) is to generate specialized cells to replace damaged tissue in patients suffering from. Human embryonic stem (hES) cells must be monitored and cared for in of differentiation is normal and expected in stem cell cultures, the.
Cells should have clean, defined edges, with little to no differentiation. The cells shown in Figure 2B are ready for passaging, and the cells shown in the Figure 2C are overcrowded. Figure 1. Differentiation in hES cultures. A differentiation of a colony B differentiation between colonies. Figure 2. Morphologies seen in hES cultures. A good cell morphology B hES cells that are ready for passaging C overcrowded cells.
1. HURRY UP AND WAIT
Sterility must be maintained at all times when working with hES cells. Clean and sterilize the biological safety cabinet and all equipment before use. All reagents must be filtered using a 0. The use of antiboiotics in hES cell culture is not necessary and should be avoided.
Dissociate the cells into small clusters cells by gentle pipetting. Although considerable progress has been made in the design of chemically defined xeno-free media, cell passage, and fold-expansion may be limited by the quality of culture supplements and their stability in culture. No official data were ever published. Although it is possible to apply GMP to animal components by careful risk assessment in certain circumstance if no better options are available , an animal xeno -free and ultimately chemically defined culture system is preferable. If the same is true for human embryonic stem cells, researchers said, then scientists may face unexpected challenges as they try to turn the controversial cells into treatments for various degenerative conditions. Acknowledgments The authors would like to thank Stephanie Ploskonka for editing the manuscript.
A separate environment should be set up as a designated picking station. The sterile enclosure for the station can be a static enclosure such as a PCR workstation with a UV light source, a laminar flow hood, or a biological safety cabinet. A dissecting microscope inside the picking station is needed to observe the colonies as the differentiated areas are removed. The front glass panel of a static enclosure or a biological safety cabinet must be modified to allow for the oculars of the microscope to extend through the panel without compromising proper airflow and sterility.
To maintain healthy hES cell cultures, cells must be passaged at the optimal time, typically every days. At this time the colonies have reached their maximum size and may be beginning to merge. Merged colonies can increase the rate of differentiation in the culture.
Split ratios generally fall between and , depending on the number of colonies plated, expansion rate, and culture conditions.
- Preventions the Sugar Solution Cookbook: More Than 200 Delicious Recipes to Balance Your Blood Sugar Naturally
- Data Mining With Neural Networks: Solving Business Problems from Application Development to Decision Support
- Meaning in the history of English : words and texts in context
- Cosmology for string theorists, a crash course (2002-05)
- The Pursuit of Signs. Semiotics, Literature, Deconstruction (Routledge Classics)